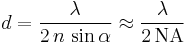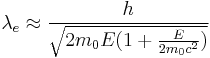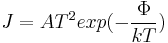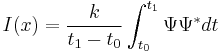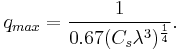Transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through. An image is formed from the interaction of the electrons transmitted through the specimen; the image is magnified and focused onto an imaging device, such as a fluorescent screen, on a layer of photographic film, or to be detected by a sensor such as a CCD camera.
TEMs are capable of imaging at a significantly higher resolution than light microscopes, owing to the small de Broglie wavelength of electrons. This enables the instrument's user to examine fine detail—even as small as a single column of atoms, which is tens of thousands times smaller than the smallest resolvable object in a light microscope. TEM forms a major analysis method in a range of scientific fields, in both physical and biological sciences. TEMs find application in cancer research, virology, materials science as well as pollution and semiconductor research.
At smaller magnifications TEM image contrast is due to absorption of electrons in the material, due to the thickness and composition of the material. At higher magnifications complex wave interactions modulate the intensity of the image, requiring expert analysis of observed images. Alternate modes of use allow for the TEM to observe modulations in chemical identity, crystal orientation, electronic structure and sample induced electron phase shift as well as the regular absorption based imaging.
The first TEM was built by Max Knoll and Ernst Ruska in 1931, with this group developing the first TEM with resolving power greater than that of light in 1933 and the first commercial TEM in 1939.
Contents |
History
Initial development


Ernst Abbe originally proposed that the ability to resolve detail in an object was limited by the wavelength of the light used in imaging, thus limiting the useful obtainable magnification from an optical microscope to a few micrometers. Developments into ultraviolet (UV) microscopes, led by Koehler, allowed for an increase in resolving power of about a factor of two. However this required more expensive quartz optical components, due to the absorption of UV by glass. At this point it was believed that obtaining an image with sub-micrometer information was simply impossible due to this wavelength constraint.[2]
It had earlier been recognized by Plücker in 1858 that the deflection of "cathode rays" (electrons) was possible by the use of magnetic fields.[3] This effect had been utilised to build primitive cathode ray oscilloscopes (CROs) as early as 1897 by Ferdinand Braun, intended as a measurement device.[4] Indeed in 1891 it was recognized by Riecke that the cathode rays could be focused by these magnetic fields, allowing for simple lens designs. Later this theory was extended by Hans Busch in his work published in 1926, who showed that the lens maker's equation, could under appropriate assumptions, be applicable to electrons.[5]
In 1928, at the Technological University of Berlin Adolf Matthias, Professor of High voltage Technology and Electrical Installations, appointed Max Knoll to lead a team of researchers to advance the CRO design. The team consisted of several PhD students including Ernst Ruska and Bodo von Borries. This team of researchers concerned themselves with lens design and CRO column placement, which they attempted to obtain the parameters that could be optimised to allow for construction of better CROs, as well as the development of electron optical components which could be used to generate low magnification (nearly 1:1) images. In 1931 the group successfully generated magnified images of mesh grids placed over the anode aperture. The device used two magnetic lenses to achieve higher magnifications, arguably the first electron microscope. In that same year, Reinhold Rudenberg, the scientific director of the Siemens company, had patented an electrostatic lens electron microscope.[2][6]
Improving resolution
At this time the wave nature of electrons, which were considered charged matter particles, had not been fully realised until the publication of the De Broglie hypothesis in 1927.[7] The group was unaware of this publication until 1932, where it was quickly realized that the De Broglie wavelength of electrons was many orders of magnitude smaller than that for light, theoretically allowing for imaging at atomic scales. In April 1932, Ruska suggested the construction of a new electron microscope for direct imaging of specimens inserted into the microscope, rather than simple mesh grids or images of apertures. With this device successful diffraction and normal imaging of aluminium sheet was achieved, however exceeding the magnification achievable with light microscopy had still not been successfully demonstrated. This goal was achieved in September 1933, using images of cotton fibers, which were quickly acquired before being damaged by the electron beam.[2]
At this time, interest in the electron microscope had increased, with other groups, such as Albert Prebus and James Hillier at the University of Toronto who constructed the first TEM in north America in 1938,[8] continually advancing TEM design.
Research continued on the electron microscope at Siemens in 1936, the aim of the research was the development improvement of TEM imaging properties, particularly with regard to biological specimens. At this time electron microscopes were being fabricated for specific groups, such as the "EM1" device used at the UK National Physical Laboratory.[9] In 1939 the first commercial electron microscope, pictured, was installed in the Physics department of I. G Farben-Werke. Further work on the electron microscope was hampered by the destruction of a new laboratory constructed at Siemens by an air-raid, as well as the death of two of the researchers, Heinz Müller and Friedrick Krause during World War II.[10]
Further research
After World War II, Ruska resumed work at Siemens, where he continued to develop the electron microscope, producing the first microscope with 100k magnification.[10] The fundamental structure of this microscope design, with multi-stage beam preparation optics, is still used in modern microscopes. The worldwide electron microscopy community advanced with electron microscopes being manufactured in Manchester UK, the USA (RCA), Germany (Siemens) and Japan . The first international conference in electron microscopy was in Delft in 1942, with more than one hundred attendees.[9] Later conferences included the "First" international conference in Paris, 1950 and then in London in 1954.
With the development of TEM, the associated technique of scanning transmission electron microscopy (STEM) was re-investigated and did not become developed until the 1970s, with Albert Crewe at the University of Chicago developing the field emission gun[11] and adding a high quality objective lens to create the modern STEM. Using this design, Crewe demonstrated the ability to image atoms using annular dark-field imaging. Crewe and coworkers at the University of Chicago developed the cold field electron emission source and built a STEM able to visualize single heavy atoms on thin carbon substrates.[12]
Background
Electrons
Theoretically, the maximum resolution, d, that one can obtain with a light microscope has been limited by the wavelength of the photons that are being used to probe the sample, λ and the numerical aperture of the system, NA.[13]
Early twentieth century scientists theorised ways of getting around the limitations of the relatively large wavelength of visible light (wavelengths of 400–700 nanometers) by using electrons. Like all matter, electrons have both wave and particle properties (as theorized by Louis-Victor de Broglie), and their wave-like properties mean that a beam of electrons can be made to behave like a beam of electromagnetic radiation. The wavelength of electrons is found by equating the de Broglie equation to the kinetic energy of an electron. An additional correction must be made to account for relativistic effects, as in a TEM an electron's velocity approaches the speed of light, c.[14]
where, h is Planck's constant, m0 is the rest mass of an electron and E is the energy of the accelerated electron. Electrons are usually generated in an electron microscope by a process known as thermionic emission from a filament, usually tungsten, in the same manner as a light bulb, or alternatively by field electron emission.[15] The electrons are then accelerated by an electric potential (measured in volts) and focused by electrostatic and electromagnetic lenses onto the sample. The transmitted beam contains information about electron density, phase and periodicity; this beam is used to form an image.
Source formation
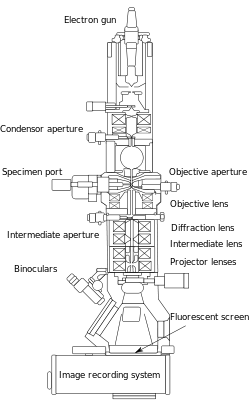

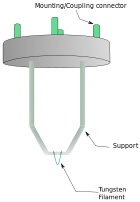
From the top down, the TEM consists of an emission source, which may be a tungsten filament, or a lanthanum hexaboride (LaB6) source.[16] For tungsten, this will be of the form of either a hairpin-style filament, or a small spike-shaped filament. LaB6 sources utilize small single crystals. By connecting this gun to a high voltage source (typically ~100-300 kV) the gun will, given sufficient current, begin to emit electrons either by thermionic or field electron emission into the vacuum. This extraction is usually aided by the use of a Wehnelt cylinder. Once extracted, the upper lenses of the TEM allow for the formation of the electron probe to the desired size and location for later interaction with the sample.[17]
Manipulation of the electron beam is performed using two physical effects. The interaction of electrons with a magnetic field will cause electrons to move according to the right hand rule, thus allowing for electromagnets to manipulate the electron beam. The use of magnetic fields allows for the formation of a magnetic lens of variable focusing power, the lens shape originating due to the distribution of magnetic flux. Additionally, electrostatic fields can cause the electrons to be deflected through a constant angle. Coupling of two deflections in opposing directions with a small intermediate gap allows for the formation of a shift in the beam path, this being used in TEM for beam shifting, subsequently this is extremely important to STEM. From these two effects, as well as the use of an electron imaging system, sufficient control over the beam path is possible for TEM operation. The optical configuration of a TEM can be rapidly changed, unlike that for an optical microscope, as lenses in the beam path can be enabled, have their strength changed, or be disabled entirely simply via rapid electrical switching, the speed of which is limited by effects such as the magnetic hysteresis of the lenses.
Optics
The lenses of a TEM allow for beam convergence, with the angle of convergence as a variable parameter, giving the TEM the ability to change magnification simply by modifying the amount of current that flows through the coil, quadrupole or hexapole lenses. The quadrupole lens is an arrangement of electromagnetic coils at the vertices of the square, enabling the generation of a lensing magnetic fields, the hexapole configuration simply enhances the lens symmetry by using six, rather than four coils.
Typically a TEM consists of three stages of lensing. The stages are the condensor lenses, the objective lenses, and the projector lenses. The condensor lenses are responsible for primary beam formation, whilst the objective lenses focus the beam down onto the sample itself. The projector lenses are used to expand the beam onto the phosphor screen or other imaging device, such as film. The magnification of the TEM is due to the ratio of the distances between the specimen and the objective lens' image plane.[18] Additional quad or hexapole lenses allow for the correction of asymmetrical beam distortions, known as astigmatism. It is noted that TEM optical configurations differ significantly with implementation, with manufacturers using custom lens configurations, such as in spherical aberration corrected instruments,[17] or TEMs utilising energy filtering to correct electron chromatic aberration.
Display
Imaging systems in a TEM consist of a phosphor screen, which may be made of fine (10-100 μm) particulate zinc sulphide, for direct observation by the operator. Optionally, an image recording system such as film based or doped YAG screen coupled CCDs.[19] Typically these devices can be removed or inserted into the beam path by the operator as required.
Components

A TEM is composed of several components, which include a vacuum system in which the electrons travel, an electron emission source for generation of the electron stream, a series of electromagnetic lenses, as well as electrostatic plates. The latter two allow the operator to guide and manipulate the beam as required. Also required is a device to allow the insertion into, motion within, and removal of specimens from the beam path. Imaging devices are subsequently used to create an image from the electrons that exit the system.
Vacuum system
To increase the mean free path of the electron gas interaction, a standard TEM is evacuated to low pressures, typically on the order of 10−4 Pa.[20] The need for this is twofold: first the allowance for the voltage difference between the cathode and the ground without generating an arc, and secondly to reduce the collision frequency of electrons with gas atoms to negligible levels—this effect is characterised by the mean free path. TEM components such as specimen holders and film cartridges must be routinely inserted or replaced requiring a system with the ability to re-evacuate on a regular basis. As such, TEMs are equipped with multiple pumping systems and airlocks and are not permanently vacuum sealed.
The vacuum system for evacuating a TEM to an operating pressure level consists of several stages. Initially a low or roughing vacuum is achieved with either a rotary vane pump or diaphragm pumps bringing the TEM to a sufficiently low pressure to allow the operation of a turbomolecular or diffusion pump which brings the TEM to its high vacuum level necessary for operations. To allow for the low vacuum pump to not require continuous operation, while continually operating the turbomolecular pumps, the vacuum side of a low-pressure pump may be connected to chambers which accommodate the exhaust gases from the turbomolecular pump.[21] Sections of the TEM may be isolated by the use of gate valves, to allow for different vacuum levels in specific areas, such as a higher vacuum of 10−4 to 10−7 Pa or higher in the electron gun in high resolution or field emission TEMs.
High-voltage TEMs require ultra high vacuums on the range of 10−7 to 10−9 Pa to prevent generation of an electrical arc, particularly at the TEM cathode.[22] As such for higher voltage TEMs a third vacuum system may operate, with the gun isolated from the main chamber either by use of gate valves or by the use of a differential pumping aperture. The differential pumping aperture is a small hole that prevents diffusion of gas molecules into the higher vacuum gun area faster than they can be pumped out. For these very low pressures either an ion pump or a getter material is used.
Poor vacuum in a TEM can cause several problems, from deposition of gas inside the TEM onto the specimen as it is being viewed through a process known as electron beam induced deposition, or in more severe cases damage to the cathode from an electrical discharge [22]. Vacuum problems due to specimen sublimation are limited by the use of a cold trap to adsorb sublimated gases in the vicinity of the specimen.[21]
Specimen stage

TEM specimen stage designs include airlocks to allow for insertion of the specimen holder into the vacuum with minimal increase in pressure in other areas of the microscope. The specimen holders are adapted to hold a standard size of grid upon which the sample is placed or a standard size of self-supporting specimen. Standard TEM grid sizes is a 3.05 mm diameter ring, with a thickness and mesh size ranging from a few to 100 μm. The sample is placed onto the inner meshed area having diameter of approximately 2.5 mm. Usual grid materials are copper, molybdenum, gold or platinum. This grid is placed into the sample holder which is paired with the specimen stage. A wide variety of designs of stages and holders exist, depending upon the type of experiment being performed. In addition to 3.05 mm grids, 2.3 mm grids are sometimes, if rarely, used. These grids were particularly used in the mineral sciences where a large degree of tilt can be required and where specimen material may be extremely rare. Electron transparent specimens have a thickness around 100 nm, but this value depends on the accelerating voltage.
Once inserted into a TEM, the sample often has to be manipulated to present the region of interest to the beam, such as in single grain diffraction, in a specific orientation. To accommodate this, the TEM stage includes mechanisms for the translation of the sample in the XY plane of the sample, for Z height adjustment of the sample holder, and usually for at least one rotation degree of freedom for the sample. Thus a TEM stage may provide four degrees of freedom for the motion of the specimen. Most modern TEMs provide the ability for two orthogonal rotation angles of movement with specialized holder designs called double-tilt sample holders. Of note however is that some stage designs, such as top-entry or vertical insertion stages once common for high resolution TEM studies, may simply only have X-Y translation available. The design criteria of TEM stages are complex, owing to the simultaneous requirements of mechanical and electron-optical constraints and have thus generated many unique implementations.
A TEM stage is required to have the ability to hold a specimen and be manipulated to bring the region of interest into the path of the electron beam. As the TEM can operate over a wide range of magnifications, the stage must simultaneously be highly resistant to mechanical drift, with drift requirements as low as a few nm/minute while being able to move several um/minute, with repositioning accuracy on the order of nanometers.[23] Earlier designs of TEM accomplished this with a complex set of mechanical downgearing devices, allowing the operator to finely control the motion of the stage by several rotating rods. Modern devices may use electrical stage designs, using screw gearing in concert with stepper motors, providing the operator with a computer-based stage input, such as a joystick or trackball.
Two main designs for stages in a TEM exist, the side-entry and top entry version.[19] Each design must accommodate the matching holder to allow for specimen insertion without either damaging delicate TEM optics or allowing gas into TEM systems under vacuum.

The most common is the side entry holder, where the specimen is placed near the tip of a long metal (brass or stainless steel) rod, with the specimen placed flat in a small bore. Along the rod are several polymer vacuum rings to allow for the formation of a vacuum seal of sufficient quality, when inserted into the stage. The stage is thus designed to accommodate the rod, placing the sample either in between or near the objective lens, dependent upon the objective design. When inserted into the stage, the side entry holder has its tip contained within the TEM vacuum, and the base is presented to atmosphere, the airlock formed by the vacuum rings.
Insertion procedures for side entry TEM holders typically involve the rotation of the sample to trigger micro switches that initiate evacuation of the airlock before the sample is inserted into the TEM column.
The second design is the top-entry holder consists of a cartridge that is several cm long with a bore drilled down the cartridge axis. The specimen is loaded into the bore, possibly utilising a small screw ring to hold the sample in place. This cartridge is inserted into an airlock with the bore perpendicular to the TEM optic axis. When sealed, the airlock is manipulated to push the cartridge such that the cartridge falls into place, where the bore hole becomes aligned with the beam axis, such that the beam travels down the cartridge bore and into the specimen. Such designs are typically unable to be tilted without blocking the beam path or interfering with the objective lens.[19]
Electron gun
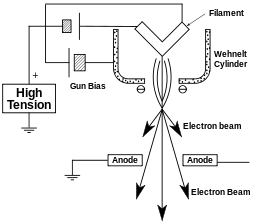
The electron gun is formed from several components: the filament, a biasing circuit, a Wehnelt cap, and an extraction anode. By connecting the filament to the negative component power supply, electrons can be "pumped" from the electron gun to the anode plate, and TEM column, thus completing the circuit. The gun is designed to create a beam of electrons exiting from the assembly at some given angle, known as the gun divergence semiangle, α. By constructing the Wehnelt cylinder such that it has a higher negative charge than the filament itself, electrons that exit the filament in a diverging manner are, under proper operation, forced into a converging pattern the minimum size of which is the gun crossover diameter.
The thermionic emission current density, J, can be related to the work function of the emitting material and is a Boltzmann distribution given below, where A is a constant, Φ is the work function and T is the temperature of the material.[19]
This equation shows that in order to achieve sufficient current density it is necessary to heat the emitter, taking care not to cause damage by application of excessive heat, for this reason materials with either a high melting point, such as tungsten, or those with a low work function (LaB6) are required for the gun filament.[24] Furthermore both lanthanum hexaboride and tungsten thermionic sources must be heated in order to achieve thermionic emission, this can be achieved by the use of a small resistive strip. To prevent thermal shock, there is often a delay enforced in the application of current to the tip, to prevent thermal gradients from damaging the filament, the delay is usually a few seconds for LaB6, and significantly lower for tungsten.
Electron lens
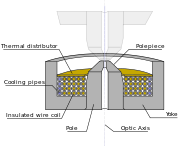
Electron lenses are designed to act in a manner emulating that of an optical lens, by focusing parallel rays at some constant focal length. Lenses may operate electrostatically or magnetically. The majority of electron lenses for TEM utilise electromagnetic coils to generate a convex lens. For these lenses the field produced for the lens must be radially symmetric, as deviation from the radial symmetry of the magnetic lens causes aberrations such as astigmatism, and worsens spherical and chromatic aberration. Electron lenses are manufactured from iron, iron-cobalt or nickel cobalt alloys,[25] such as permalloy. These are selected for their magnetic properties, such as magnetic saturation, hysteresis and permeability.
The components include the yoke, the magnetic coil, the poles, the polepiece, and the external control circuitry. The polepiece must be manufactured in a very symmetrical manner, as this provides the boundary conditions for the magnetic field that forms the lens. Imperfections in the manufacture of the polepiece can induce severe distortions in the magnetic field symmetry, which induce distortions that will ultimately limit the lenses' ability to reproduce the object plane. The exact dimensions of the gap, pole piece internal diameter and taper, as well as the overall design of the lens is often performed by finite element analysis of the magnetic field, whilst considering the thermal and electrical constraints of the design.[25]
The coils which produce the magnetic field are located within the lens yoke. The coils can contain a variable current, but typically utilise high voltages, and therefore require significant insulation in order to prevent short-circuiting the lens components. Thermal distributors are placed to ensure the extraction of the heat generated by the energy lost to resistance of the coil windings. The windings may be water cooled, using a chilled water supply in order to facilitate the removal of the high thermal duty.
Apertures
Apertures are annular metallic plates, through which electrons that are further than a fixed distance from the optic axis may be excluded. These consist of a small metallic disc that is sufficiently thick to prevent electrons from passing through the disc, whilst permitting axial electrons. This permission of central electrons in a TEM causes two effects simultaneously: firstly, apertures decrease the beam intensity as electrons are filtered from the beam, which may be desired in the case of beam sensitive samples. Secondly, this filtering removes electrons that are scattered to high angles, which may be due to unwanted processes such as spherical or chromatic aberration, or due to diffraction from interaction within the sample.[26]
Apertures are either a fixed aperture within the column, such as at the condensor lens, or are a movable aperture, which can be inserted or withdrawn from the beam path, or moved in the plane perpendicular to the beam path. Aperture assemblies are mechanical devices which allow for the selection of different aperture sizes, which may be used by the operator to trade off intensity and the filtering effect of the aperture. Aperture assemblies are often equipped with micrometers to move the aperture, required during optical calibration.
Imaging methods
Imaging methods in TEM utilize the information contained in the electron waves exiting from the sample to form an image. The projector lenses allow for the correct positioning of this electron wave distribution onto the viewing system. The observed intensity of the image, I, assuming sufficiently high quality of imaging device, can be approximated as proportional to the time-average amplitude of the electron wavefunctions, where the wave which form the exit beam is denoted by Ψ.[27]
Different imaging methods therefore attempt to modify the electron waves exiting the sample in a form that is useful to obtain information with regards to the sample, or beam itself. From the previous equation, it can be deduced that the observed image depends not only on the amplitude of beam, but also on the phase of the electrons, although phase effects may often be ignored at lower magnifications. Higher resolution imaging requires thinner samples and higher energies of incident electrons. Therefore the sample can no longer be considered to be absorbing electrons, via a Beer's law effect, rather the sample can be modelled as an object that does not change the amplitude of the incoming electron wavefunction. Rather the sample modifies the phase of the incoming wave; this model is known as a pure phase object, for sufficiently thin specimens phase effects dominate the image, complicating analysis of the observed intensities.[27] For example, to improve the contrast in the image the TEM may be operated at a slight defocus to enhance contrast, owing to convolution by the contrast transfer function of the TEM[28], which would normally decrease contrast if the sample was not a weak phase object.
Contrast formation
Contrast formation in the TEM depends greatly on the mode of operation. Complex imaging techniques, which utilise the unique ability to change lens strength or to deactivate a lens, allow for many operating modes. These modes may be used to discern information that is of particular interest to the investigator.
Bright field
The most common mode of operation for a TEM is the bright field imaging mode. In this mode the contrast formation, when considered classically, is formed directly by occlusion and absorption of electrons in the sample. Thicker regions of the sample, or regions with a higher atomic number will appear dark, whilst regions with no sample in the beam path will appear bright – hence the term "bright field". The image is in effect assumed to be a simple two dimensional projection of the sample down the optic axis, and to a first approximation may be modelled via Beer's law[13], more complex analyses require the modelling of the sample to include phase information.[27]
Diffraction contrast

Samples can exhibit diffraction contrast, whereby the electron beam undergoes Bragg scattering, which in the case of a crystalline sample, disperses electrons into discrete locations in the back focal plane. By the placement of apertures in the back focal plane, i.e. the objective aperture, the desired Bragg reflections can be selected (or excluded), thus only parts of the sample that are causing the electrons to scatter to the selected reflections will end up projected onto the imaging apparatus.
If the reflections that are selected do not include the unscattered beam (which will appear up at the focal point of the lens), then the image will appear dark wherever no sample scattering to the selected peak is present, as such a region without a specimen will appear dark. This is known as a dark-field image.
Modern TEMs are often equipped with specimen holders that allow the user to tilt the specimen to a range of angles in order to obtain specific diffraction conditions, and apertures placed above the specimen allow the user to select electrons that would otherwise be diffracted in a particular direction from entering the specimen.
Applications for this method include the identification of lattice defects in crystals. By carefully selecting the orientation of the sample, it is possible not just to determine the position of defects but also to determine the type of defect present. If the sample is oriented so that one particular plane is only slightly tilted away from the strongest diffracting angle (known as the Bragg Angle), any distortion of the crystal plane that locally tilts the plane to the Bragg angle will produce particularly strong contrast variations. However, defects that produce only displacement of atoms that do not tilt the crystal to the Bragg angle (i. e. displacements parallel to the crystal plane) will not produce strong contrast.[29]
Electron energy loss
Utilizing the advanced technique of EELS, for TEMs appropriately equipped electrons can be rejected based upon their voltage (which, due to constant charge is their energy), using magnetic sector based devices known as EELS spectrometers. These devices allow for the selection of particular energy values, which can be associated with the way the electron has interacted with the sample. For example different elements in a sample result in different electron energies in the beam after the sample. This normally results in chromatic aberration – however this effect can, for example, be used to generate an image which provides information on elemental composition, based upon the atomic transition during electron-electron interaction.[30]
EELS spectrometers can often be operated in both spectroscopic and imaging modes, allowing for isolation or rejection of elastically scattered beams. As for many images inelastic scattering will include information that may not be of interest to the investigator thus reducing observable signals of interest, EELS imaging can be used to enhance contrast in observed images, including both bright field and diffraction, by rejecting unwanted components.
Phase contrast
Crystal structure can also be investigated by High Resolution Transmission Electron Microscopy (HRTEM), also known as phase contrast. When utilizing a Field emission source, of uniform thickness, the images are formed due to differences in phase of electron waves, which is caused by specimen interaction.[28] Image formation is given by the complex modulus of the incoming electron beams. As such, the image is not only dependent on the number of electrons hitting the screen, making direct interpretation of phase contrast images more complex. However this effect can be used to an advantage, as it can be manipulated to provide more information about the sample, such as in complex phase retrieval techniques.
Diffraction

As previously stated, by adjusting the magnetic lenses such that the back focal plane of the lens rather than the imaging plane is placed on the imaging apparatus a diffraction pattern can be generated. For thin crystalline samples, this produces an image that consists of a pattern of dots in the case of a single crystal, or a series of rings in the case of a polycrystalline or amorphous solid material. For the single crystal case the diffraction pattern is dependent upon the orientation of the specimen and the structure of the sample illuminated by the electron beam. This image provides the investigator with information about the space group symmetries in the crystal and the crystal's orientation to the beam path. This is typically done without utilising any information but the position at which the diffraction spots appear and the observed image symmetries.
Diffraction patterns can have a large dynamic range, and for crystalline samples, may have intensities greater than those recordable by CCD. As such, TEMs may still be equipped with film cartridges for the purpose of obtaining these images, as the film is a single use detector.

Analysis of diffraction patterns beyond point-position can be complex, as the image is sensitive to a number of factors such as specimen thickness and orientation, objective lens defocus, spherical and chromatic aberration. Although quantitative interpretation of the contrast shown in lattice images is possible, it is inherently complicated and can require extensive computer simulation and analysis, such as electron multislice analysis.[31]
More complex behaviour in the diffraction plane is also possible, with phenomena such as Kikuchi lines arising from multiple diffraction within the crystalline lattice. In convergent beam electron diffraction (CBED) where a non-parallel, i.e. converging, electron wavefront is produced by concentrating the electron beam into a fine probe at the sample surface, the interaction of the convergent beam can provide information beyond structural data such as sample thickness.
Three dimensional imaging
As TEM specimen holders typically allow for the rotation of a sample by a desired angle, multiple views of the same specimen can be obtained by rotating the angle of the sample along an axis perpendicular to the beam. By taking multiple images of a single TEM sample at differing angles, typically in 1° increments, a set of images known as a "tilt series" can be collected. Under purely absorption contrast conditions, this set of images can be used to construct a three-dimensional representation of the sample.[33]
The reconstruction is accomplished by a two-step process, first images are aligned to account for errors in the positioning of a sample; such errors can occur due to vibration or mechanical drift. Alignment methods use image registration algorithms, such as autocorrelation methods to correct these errors. Secondly, using a technique known as filtered back projection, the aligned image slices can be transformed from a set of two-dimensional images, Ij(x,y), to a single three-dimensional image, I'j(x,y,z). This three dimensional image is of particular interest when morphological information is required, further study can be undertaken using computer algorithms, such as isosurfaces and data slicing to analyse the data.
As TEM samples cannot typically be viewed at a full 180° rotation, the observed images typically suffer from a "missing wedge" of data, which when using Fourier based back projection methods decreases the range of resolvable frequencies in the three dimensional reconstruction.[33] Mechanical techniques, such as multi-axis tilting, as well as numerical techniques exist to limit the impact of this missing data on the observed specimen morphology. Variants on this method, referred to as single particle analysis, use images of multiple identical objects at different orientations to produce the image data required for three dimensional reconstruction. Assuming that objects do not have significant preferred orientations, this method does not suffer from the missing data wedge, however it assumes that the different objects imaged can be treated as if the data was generated from a single object.
Sample preparation
Sample preparation in TEM can be a complex procedure. TEM specimens are required to be at most hundreds of nanometers thick, as unlike neutron or X-Ray radiation the electron beam interacts readily with the sample, an effect that increases roughly with atomic number squared (z2).[13] High quality samples will have a thickness that is comparable to the mean free path of the electrons that travel through the samples, which may be only a few tens of nanometers. Preparation of TEM specimens is specific to the material under analysis and the desired information to obtain from the specimen. As such, many generic techniques have been used for the preparation of the required thin sections.
Materials that have dimensions small enough to be electron transparent, such as powders or nanotubes, can be quickly prepared by the deposition of a dilute sample containing the specimen onto support grids or films. In the biological sciences in order to withstand the instrument vacuum and facilitate handling, biological specimens can be fixated using either a negative staining material such as uranyl acetate or by plastic embedding. Alternately samples may be held at liquid nitrogen temperatures after embedding in vitreous ice.[34] In material science and metallurgy the specimens tend to be naturally resistant to vacuum, but still must be prepared as a thin foil, or etched so some portion of the specimen is thin enough for the beam to penetrate. Constraints on the thickness of the material may be limited by the scattering cross-section of the atoms from which the material is comprised.
Tissue sectioning
By passing samples over a glass or diamond edge, small, thin sections can be readily obtained using a semi-automated method.[35] This method is used to obtain thin, minimally deformed samples that allow for the observation of tissue samples. Additionally inorganic samples have been studied, such as aluminium, although this usage is limited owing to the heavy damage induced in the less soft samples.[36] To prevent charge build-up at the sample surface, tissue samples need to be coated with a thin layer of conducting material, such as carbon, where the coating thickness is several nanometers. This may be achieved via an electric arc deposition process using a sputter coating device.
Sample staining

Details in light microscope samples can be enhanced by stains that absorb light; similarly TEM samples of biological tissues can utilize high atomic number stains to enhance contrast. The stain absorbs electrons or scatters part of the electron beam which otherwise is projected onto the imaging system. Compounds of heavy metals such as osmium, lead, or uranium may be used prior to TEM observation to selectively deposit electron dense atoms in or on the sample in desired cellular or protein regions, requiring an understanding of how heavy metals bind to biological tissues.
Mechanical milling
Mechanical polishing may be used to prepare samples. Polishing needs to be done to a high quality, to ensure constant sample thickness across the region of interest. A diamond, or cubic boron nitride polishing compound may be used in the final stages of polishing to remove any scratches that may cause contrast fluctuations due to varying sample thickness. Even after careful mechanical milling, additional fine methods such as ion etching may be required to perform final stage thinning.
Chemical etching
Certain samples may be prepared by chemical etching, particularly metallic specimens. These samples are thinned using a chemical etchant, such as an acid, to prepare the sample for TEM observation. Devices to control the thinning process may allow the operator to control either the voltage or current passing through the specimen, and may include systems to detect when the sample has been thinned to a sufficient level of optical transparency.
Ion etching

Ion etching is a sputtering process that can remove very fine quantities of material. This is used to perform a finishing polish of specimens polished by other means. Ion etching uses an inert gas passed through an electric field to generate a plasma stream that is directed to the sample surface. Acceleration energies for gases such as argon are typically a few kilovolts. The sample may be rotated to promote even polishing of the sample surface. The sputtering rate of such methods is on the order of tens of micrometers per hour, limiting the method to only extremely fine polishing.
More recently focussed ion beam methods have been used to prepare samples. FIB is a relatively new technique to prepare thin samples for TEM examination from larger specimens. Because FIB can be used to micro-machine samples very precisely, it is possible to mill very thin membranes from a specific area of interest in a sample, such as a semiconductor or metal. Unlike inert gas ion sputtering, FIB makes use of significantly more energetic gallium ions and may alter the composition or structure of the material through gallium implantation.[37]
Modifications
The capabilities of the TEM can be further extended by additional stages and detectors, sometimes incorporated on the same microscope. An electron cryomicroscope (CryoTEM) is a TEM with a specimen holder capable of maintaining the specimen at liquid nitrogen or liquid helium temperatures. This allows imaging specimens prepared in vitreous ice, the preferred preparation technique for imaging individual molecules or macromolecular assemblies.[38]
A TEM can be modified into a scanning transmission electron microscope (STEM) by the addition of a system that rasters the beam across the sample to form the image, combined with suitable detectors. Scanning coils are used to deflect the beam, such as by an electrostatic shift of the beam, where the beam is then collected using a current detector such as a faraday cup, which acts as a direct electron counter. By correlating the electron count to the position of the scanning beam (known as the "probe"), the transmitted component of the beam may be measured. The non-transmitted components may be obtained either by beam tilting or by the use of annular dark field detectors.
In-situ experiments may also be conducted with experiments such as in-situ reactions or material deformation testing.[39]
Modern research TEMs may include aberration correctors,[17] to reduce the amount of distortion in the image. Incident beam Monochromators may also be used which reduce the energy spread of the incident electron beam to less than 0.15 eV.[17] Major TEM makers include JEOL, Hitachi High-technologies, FEI Company (from merging with Philips Electron Optics), Carl Zeiss and NION.
Low voltage electron microscope (LVEM)
The low voltage electron microscope (LVEM) is a combination of SEM, TEM and STEM in one instrument, which operated at relatively low electron accelerating voltage of 5 kV. Low voltage increases image contrast which is especially important for biological specimens. This increase in contrast significantly reduces, or even eliminates the need to stain. Sectioned samples generally need to be thinner than they would be for conventional TEM (20-65 nm). Resolutions of a few nm are possible in TEM, SEM and STEM modes.[40][41]
Limitations
There are a number of drawbacks to the TEM technique. Many materials require extensive sample preparation to produce a sample thin enough to be electron transparent, which makes TEM analysis a relatively time consuming process with a low throughput of samples. The structure of the sample may also be changed during the preparation process. Also the field of view is relatively small, raising the possibility that the region analysed may not be characteristic of the whole sample. There is potential that the sample may be damaged by the electron beam, particularly in the case of biological materials.
Resolution limits
The limit of resolution obtainable in a TEM may be described in several ways, and is typically referred to as the information limit of the microscope. One commonly used value is a cut-off value of the contrast transfer function, a function that is usually quoted in the frequency domain to define the reproduction of spatial frequencies of objects in the object plane by the microscope optics. A cut-off frequency, qmax, for the transfer function may be approximated with the following equation, where Cs is the spherical aberration coefficient and λ is the electron wavelength:[26]
For a 200 kV microscope, with partly corrected spherical aberrations ("to the third order") and a Cs value of 1 µm,[42] a theoretical cut-off value might be 1/qmax = 42 pm [26]. The same microscope without a corrector would have Cs = 0.5 mm and thus a 200-pm cut-off.[42] Practically, the spherical aberrations are suppressed in the best, "aberration-corrected" microscopes. Their resolution is however limited by electron source geometry and brightness and chromatic aberrations in the objective lens system.[17][43]
Intriguingly, the frequency domain representation of the contrast transfer function may often have an oscillatory nature[44], which can be tuned by adjusting the focal value of the objective lens. This oscillatory nature implies that some spatial frequencies are faithfully imaged by the microscope, whilst others are suppressed. By combining multiple images with different spatial frequencies, the use of techniques such as focal series reconstruction can be used to improve the resolution of the TEM in a limited manner.[26] The contrast transfer function can, to some extent, be experimentally approximated through techniques such as Fourier transforming images of amorphous material, such as amorphous carbon.
More recently, advances in aberration corrector design have been able to reduce spherical aberrations[45] and to achieve resolution below 0.5 Ångströms (50 pm)[43] at magnifications above 50 million times.[46] Improved resolution allows for the imaging of lighter atoms that scatter electrons less efficiently, such as lithium atoms in lithium battery materials.[47] The ability to determine the position of atoms within materials has made the HRTEM an indispensable tool for nanotechnology research and development in many fields, including heterogeneous catalysis and the development of semiconductor devices for electronics and photonics.[48]
See also
- Electron beam induced deposition
- Electron diffraction
- Electron energy loss spectroscopy (EELS)
- Electron microscope
- Energy filtered transmission electron microscopy (EFTEM)
- High-resolution transmission electron microscopy (HRTEM)
- Low Voltage Electron Microscopy (LVEM)
- Scanning confocal electron microscopy
- Scanning electron microscope (SEM)
- Scanning transmission electron microscope (STEM)
- Transmission Electron Aberration-corrected Microscope
References
- ↑ "Viruses". http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/V/Viruses.html.
- ↑ 2.0 2.1 2.2 Ernst Ruska, translation my T Mulvey. The Early Development of Electron Lenses and Electron Microscopy. ISBN 3-7776-0364-3.
- ↑ Plücker, J. (1858). "Über die Einwirkung des Magneten auf die elektrischen Entladungen in verdünnten Gasen". Poggendorffs Annalen der Physik und Chemie 103: 88–106. doi:10.1002/andp.18581790106.
- ↑ "Ferdinand Braun, The Nobel Prize in Physics 1909, Biography". http://nobelprize.org/nobel_prizes/physics/laureates/1909/braun-bio.html.
- ↑ "The Nobel Prize in Physics 1986, Perspectives - Life through a Lens". http://nobelprize.org/nobel_prizes/physics/laureates/1986/perspectives.html.
- ↑ "Configuration for the enlarged imaging of objects by electron beams". May 30, 1931. http://v3.espacenet.com/searchResults?locale=en_GB&PN=DE906737&compact=false&DB=EPODOC.
- ↑ Broglie, L. (1928). "La nouvelle dynamique des quanta". Électrons et Photons: Rapports et Discussions du Cinquième Conseil de Physique. Solvay.
- ↑ "Dr. James Hillier, Biography". http://comdir.bfree.on.ca/hillier/hilbio.htm.
- ↑ 9.0 9.1 Hawkes, P. (Ed.) (1985). The beginnings of Electron Microscopy. Academic Press.
- ↑ 10.0 10.1 "Ernst Ruska, Nobel Prize Lecture". http://nobelprize.org/nobel_prizes/physics/laureates/1986/ruska-lecture.html.
- ↑ Crewe, Albert V; Isaacson, M. and Johnson, D. (1969). "A Simple Scanning Electron Microscope". Rev. Sci. Inst. 40: 241–246. doi:10.1063/1.1683910.
- ↑ Crewe, Albert V; Wall, J. and Langmore, J. (1970). "Visibility of a single atom". Science 168 (3937): 1338–1340. doi:10.1126/science.168.3937.1338. ISSN 0036-8075. PMID 17731040.
- ↑ 13.0 13.1 13.2 Transmission Electron Microscopy and Diffractometry of Materials. Springer. 2007. ISBN 3540738851.
- ↑ Champness, P. E. (2001). Electron Diffraction in the Transmission Electron Microscope. Garland Science. ISBN 1859961479.
- ↑ Hubbard, A (1995). The Handbook of surface imaging and visualization. CRC Press. ISBN 0849389119.
- ↑ Egerton, R (2005). Physical principles of electron microscopy. Springer. ISBN 0387258000.
- ↑ 17.0 17.1 17.2 17.3 17.4 Rose, H H (2008). "Optics of high-performance electron Microscopes" (free download review on electron optics). Science and Technology of Advanced Materials 9: 014107. doi:10.1088/0031-8949/9/1/014107.
- ↑ "The objective lens of a TEM, the heart of the electron microscope". http://www.rodenburg.org/guide/t700.html.
- ↑ 19.0 19.1 19.2 19.3 Williams, D and Carter, C. B. (1996). Transmission Electron Microscopy. 1 - Basics. Plenum Press. ISBN 0-306-45324-X.
- ↑ "The Vacuum System Of a TEM". http://www.rodenburg.org/guide/t1400.html.
- ↑ 21.0 21.1 Biological Electron Microscopy: Theory, techniques and troubleshooting. springer. 2003. ISBN 030677491.
- ↑ 22.0 22.1 Chapman, S. K. (1986). Maintaining and Monitoring the Transmission Electron Microscope. Royal Microscopical Society Microscopy Handbooks. 08. Oxford University Press. ISBN 0198564074.
- ↑ Pulokas, J; Green, C; Kisseberth, N; Potter, CS; Carragher, B (Dec 1999). "Improving the Positional Accuracy of the Goniometer on the Philips CM Series TEM". Journal of Structural Biology 128 (3): 250–256. doi:10.1006/jsbi.1999.4181. ISSN 1047-8477. PMID 10633064.
- ↑ Buckingham, J (1965). "Thermionic emission properties of a lanthanum hexaboride/rhenium cathode". British Journal of Applied Physics 16: 1821.
- ↑ 25.0 25.1 Edited by Jon Orloff (1197). Orloff, J. ed. Handbook of Electron Optics. CRC-press. ISBN 0849325137.
- ↑ 26.0 26.1 26.2 26.3 Reimer,L and Kohl, H (2008). Transmission Electron Microscopy: Physics of Image Formation. Springer. ISBN 0387347585.
- ↑ 27.0 27.1 27.2 Cowley, J. M (1995). Diffraction physics. Elsevier Science B. V.. ISBN 0444822186.
- ↑ 28.0 28.1 Kirkland, E (1998). Advanced computing in Electron Microscopy. Springer. ISBN 0306459361.
- ↑ Hull, D. and Bacon, J (2001). Introduction to dislocations (4th ed.). Butterworth-Heinemann. ISBN 0750646810.
- ↑ Egerton, R. F. (1996). Electron Energy-loss Spectroscopy in the Electron Microscope. springer. ISBN 9780306452239.
- ↑ "The Scattering of Electrons by Atoms and Crystals. I. A New Theoretical Approach". Acta Crystallographica 199 (3): 609–619. 1957.
- ↑ Mast, Jan, Mast,J; Demeestere, L (Feb 2009). "Electron tomography of negatively stained complex viruses: application in their diagnosis". Diagnostic Pathology 4: 5. doi:10.1186/1746-1596-4-5. PMID 19208223. PMC 2649040. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2649040.
- ↑ 33.0 33.1 Joachim Frank, editor (2006). Frank, J. ed. Electron tomography: methods for three-dimensional visualization of structures in the cell. Springer. ISBN 9780387312347.
- ↑ Amzallag A., Vaillant C., Jacob M., Unser M., Bednar J., Kahn J., Dubochet J., Stasiak A. and John H. Maddocks, A (2006). "3D reconstruction and comparison of shapes of DNA minicircles observed by cryo-electron microscopy" (Free full text). Nucleic Acids Research 34 (18): e125. doi:10.1093/nar/gkl675. ISSN 0305-1048. PMID 17012274. PMC 1635295. http://nar.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=17012274.
- ↑ Porter, K and Blum, J (1953). "A study in Microtomy for Electron Microscopy". The anatomical record 117 (4): 685. doi:10.1002/ar.1091170403. PMID 13124776.
- ↑ Phillips (1961). "Diamond knife ultra microtomy of metals and the structure of microtomed sections". British Journal of Applied Physics 12: 554. doi:10.1088/0508-3443/12/10/308.
- ↑ Baram, M; Kaplan, WD (Dec 2008). "Quantitative HRTEM analysis of FIB prepared specimens". Journal of Microscopy 232 (3): 395–05. doi:10.1111/j.1365-2818.2008.02134.x. ISSN 0022-2720. PMID 19094016. http://www3.interscience.wiley.com/journal/121540404/abstract.
- ↑ Li, Z; Baker, ML; Jiang, W; Estes, MK; Prasad, BV (Feb 2009). "Rotavirus Architecture at Subnanometer Resolution". Journal of Virology 83 (4): 1754–1766. doi:10.1128/JVI.01855-08. ISSN 0022-538X. PMID 19036817. PMC 2643745. http://jvi.asm.org/cgi/content/full/83/4/1754?view=long&pmid=19036817.
- ↑ Haque, M. A. and Saif, M. T. A. (2001). "In-situ tensile testing of nano-scale specimens in SEM and TEM". Experimental Mechanics 42: 123. doi:10.1007/BF02411059.
- ↑ Nebesářová1, Jana; Vancová, Marie (2007). "How to Observe Small Biological Objects in Low Voltage Electron Microscope". Microscopy and Microanalysis 13 (3): 248–249. doi:10.1017/S143192760708124X (inactive 2010-03-20).
- ↑ Drummy, Lawrence, F.; Yang, Junyan; Martin, David C. (2004). "Low-voltage electron microscopy of polymer and organic molecular thin films". Ultramicroscopy 99 (4): 247–256. doi:10.1016/j.ultramic.2004.01.011. PMID 15149719.
- ↑ 42.0 42.1 Furuya, Kazuo (2008). "Nanofabrication by advanced electron microscopy using intense and focused beam" (free download pdf). Science and Technology of Advanced Materials 9: 014110. doi:10.1088/1468-6996/9/1/014110.
- ↑ 43.0 43.1 Erni, Rolf; Rossell, MD; Kisielowski, C; Dahmen, U (2009). "Atomic-Resolution Imaging with a Sub-50-pm Electron Probe". Physical Review Letters 102 (9): 096101. doi:10.1103/PhysRevLett.102.096101. PMID 19392535.
- ↑ Henning Stahlberg. "Contrast Transfer Functions". http://2dx.org/workshop/2008/speaker-pdf-notes-drop-box/Stahlberg-Sept11-2008.pdf.
- ↑ Tanaka, Nobuo (2008). "Present status and future prospects of spherical aberration corrected TEM/STEM for study of nanomaterials" (free download review). Sci. Technol. Adv. Mater. 9: 014111. doi:10.1088/1468-6996/9/1/014111.
- ↑ "The Scale of Things (Office of Basic Energy Sciences)". http://www.sc.doe.gov/bes/scale_of_things.html.
- ↑ "Imaging lithium atoms at sub-Ångström resolution". http://www.fei.com/Portals/_default/PDFs/content/2006_06_LithiumImagingOkeefe_wp.pdf.
- ↑ "Sub-Ångstrom Electron Microscopy for Sub-Ångstrom Nano-Metrology". http://www.osti.gov/bridge/servlets/purl/821768-E3YVgN/native/821768.pdf.
External links
- The National Center for Electron Microscopy, Berkeley California USA
- The National Center for Macromolecular Imaging, Houston Texas USA
- The National Resource for Automated Molecular Microscopy, La Jolla California USA
- Tutorial courses in Transmission Electron Microscopy
- Cambridge University Teaching and Learning Package on TEM
- Online course on Transmission Electron Microscopy and Crystalline Imperfections Dr. Eric Stach (2008).